Page 111 - AERB
P. 111
Large volumes of oxalic acid supernatant solution 7.4.4 Retention of Iodine in Aqueous Streams using
were generated in this process and the destruction of Novel Adsorbents
oxalic acid is essential for the recovery of plutonium in
the supernatant as well as for the safe disposal of the Radioiodine could be released from nuclear reactor
oxalate waste. systems during low probable postulated accidental
scenarios. Iodine is expected to be released into the
containment as aerosols containing metal iodides such
as CsI, AgI, InI, FeI , etc. The unique feature is that if
2
iodine can dissolve in water, it can undergo chemical
transformations to volatile chemical form which can
partition back into the containment atmosphere. For the
purpose of retaining iodine in the sump itself, sorbents
which can adsorb and retain iodine in the aqueous phase
are being employed. For this, silver coated alumina was
prepared in-house using chemical impregnation method
and characterized using techniques such as XRD, SEM
etc. Iodine removal from aqueous phase was successfully
demonstrated at different pH ranges. The iodine removal
was found to follow the order, Neutral > Acidic > Alkaline.
7.4.5 Synthesis and Thermal Characterization of
Cerium loaded Strontium Borophosphate
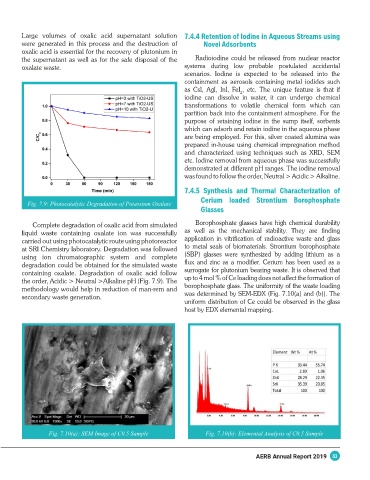
Fig. 7.9: Photocatalytic Degradation of Potassium Oxalate
Glasses
Complete degradation of oxalic acid from simulated Borophosphate glasses have high chemical durability
liquid waste containing oxalate ion was successfully as well as the mechanical stability. They are finding
carried out using photocatalytic route using photoreactor application in vitrification of radioactive waste and glass
at SRI Chemistry laboratory. Degradation was followed to metal seals of biomaterials. Strontium borophosphate
using ion chromatographic system and complete (SBP) glasses were synthesized by adding lithium as a
degradation could be obtained for the simulated waste flux and zinc as a modifier. Cerium has been used as a
containing oxalate. Degradation of oxalic acid follow surrogate for plutonium bearing waste. It is observed that
the order, Acidic > Neutral >Alkaline pH (Fig. 7.9). The up to 4 mol % of Ce loading does not affect the formation of
methodology would help in reduction of man-rem and borophosphate glass. The uniformity of the waste loading
secondary waste generation. was determined by SEM-EDX (Fig. 7.10(a) and (b)). The
uniform distribution of Ce could be observed in the glass
host by EDX elemental mapping.
Fig. 7.10(a): SEM Image of C0.5 Sample Fig. 7.10(b): Elemental Analysis of C0.5 Sample
AERB Annual Report 2019 83